Tiny structures, big impact
Miqin Zhang is working to improve cancer treatment with nanoparticles made from the same material found in crustacean shells.
By: Sarah DeWeerdt
Photos: Dennis Wise / University of Washington
Top image: Immunostained neuron stem cells.
Lots of people in Seattle enjoy seafood. But Miqin Zhang has a different sort of appreciation for shrimp and crabs than most: one of her longtime passions is chitosan, a long molecule with a backbone of carbon atoms that is a component of the hard outer skeleton of shellfish.

“Chitosan is really magical,” says Zhang, a professor of materials science and engineering and of neurological surgery. “It's very biocompatible, and it has a lot of functional groups.” These characteristics make chitosan-based materials safe to use inside the human body and offer flexibility for engineers like Zhang to manipulate the molecule in myriad ways.
Zhang uses chitosan to build nanoparticles — tiny structures barely thousandths of a hair-width in size, too small to be seen with a conventional microscope — for use in cancer therapy. A member of the Institute for Nano-Engineered Systems, she’s one of several researchers across the UW working in the rapidly growing area of nanotherapeutics — the use of nanomaterials to diagnose, treat and monitor disease.
Using chitosan to deliver anti-cancer drugs
Many chemotherapy drugs are conventionally delivered by injecting them into the bloodstream. But this results in them being washed out of the body quickly, sometimes within minutes, and requires high doses of the drugs, which can have toxic effects on healthy tissues. Nanoparticles loaded with chemotherapy drugs have the potential to solve these problems by staying in the body longer, protecting drugs from being broken down before they reach their target cells, delivering the drugs precisely to tumors, and sparing healthy tissues.
Creating nanoparticles that can do all that is far from straightforward. The chemical properties of many cancer drugs make them difficult to load into small nanoparticles, Zhang says. But her team serendipitously discovered a solution: after binding chitosan molecules to the anti-cancer drug paclitaxel and leaving them in a beaker of standard laboratory solution overnight, the researchers discovered the compounds had assembled themselves into nanofibers. When these nanofibers are injected into the body, proteins in the blood cause them to break apart into nanoparticles of just the right size.
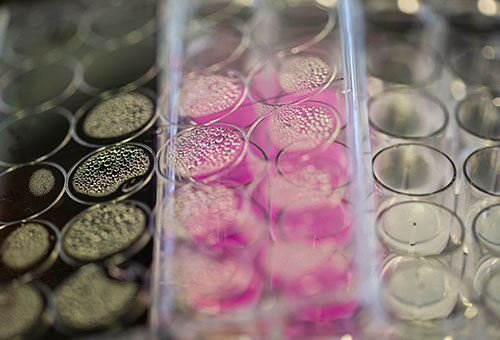
Zhang is using chitosan, a long molecule with a backbone of carbon atoms that is a component of the hard outer skeleton of shellfish, as the vehicle for delivering anti-cancer drugs more effectively.
Pictured: Culture cell plates containing tumor cells treated with nanoparticles.
In lab tests, Zhang and her team have found that paclitaxel-loaded nanoparticles inhibit mammary tumor growth and prevent its spread better than the standard formulation of paclitaxel. The nanoparticles stay in the bloodstream more than 12 times longer than the standard formulation and show no evidence of toxicity — unlike the solution used in the standard formulation, which can cause liver damage.

Zhang began working with nanomaterials over a decade ago, when the field was in its infancy. “I wanted to do something new, very new — pioneer,” she says.
The attraction to cancer medicine was more personal. Many of Zhang’s female relatives have had breast or other cancers, and two of her aunts died from the disease in their 30s, leaving young children behind. “It’s kind of painful,” she says. Working on cancer drug delivery offered a way to combine her intellectual passions with the potential to help cancer patients like her family members.
Advancing efforts with multifunctional materials
In addition to chitosan, Zhang’s team has constructed nanoparticles from a variety of other materials. “We’re particularly interested in iron oxide nanoparticles because they are biocompatible, biodegradable and detectable in magnetic resonance imaging (MRI),” she says.
This offers the opportunity to combine multiple functions in one tiny structure: both imaging and drug delivery. For example, a nanoparticle formulation with a hollow shell coating of iron oxide nanoparticles and chitosan, and cargo of the chemotherapy drug doxorubicin shrinks tumors in mice and also kills cancer cells growing in laboratory dishes, the team has reported. That effort involved a unique method of nanoparticle assembly: the researchers essentially deposited the drug into the hollow shell of the structure rather than trying to inject the fragile drug molecule into a completed particle. The iron oxide nanoparticles then triggered on-demand drug release using an external magnetic field.
Iron oxide nanoparticles show no evidence of toxicity or immune system activation in a lab study conducted by Zhang’s team. That study also demonstrated that MRI can reliably track the distribution and spread of nanoparticles in the body, providing a tool that could aid in bringing them into the clinic.


From left to right, counterclockwise: Biology student Olivia Chang examines brain tumor stem cells to determine the effect that nanoparticles have had. | Iron oxide nanoparticles of different concentrations. The light and dark colors represent low and high concentrations. | A microdevice used for producing nanoparticles of uniform size at a large scale.
Nanoparticles could be tweaked to carry all sorts of different cancer therapeutics, Zhang thinks. Another nanoparticle from her lab is designed to carry a small interfering RNA (siRNA) — a short molecule of genetic material that is designed to turn off a specific gene. In this case, the target gene is the one responsible for resistance to chemotherapy in an aggressive form of brain cancer known as glioblastoma.
The siRNA nanoparticle “can be combined with existing chemotherapy, radiation therapy and other therapy,” Zhang says — aiding in the fight against this cancer. In mice, these nanoparticles are able to cross the blood-brain barrier, home and bind to tumor cells, and reduce expression of the target gene, Zhang and her team reported this year. Mice with glioblastoma tumors treated with the combination of the nanoparticle and chemotherapy live longer than those treated with the chemotherapy drug alone.
Another nanoparticle being developed in the lab carries a cancer drug plus a molecule that stimulates the body’s immune system to fight cancer. It’s designed to home specifically to tumor cells of a kind of breast cancer known as triple negative breast cancer, as well as immune cells. Zhang’s team has recently reported that the nanoparticle inhibits tumor growth and spread, and improves survival compared to standard treatment for this difficult to treat form of breast cancer.
So far, Zhang has tested the various nanoparticles developed in her lab. But after a decade of this work, Zhang says she is ready to commercialize them and eventually launch clinical trials. “I feel that we have a really good solution now,” she says. “We have several unique nanoparticle formulations for treating different types of cancers. They are ready to go, really.”
Population health at the UW College of Engineering
Healthier communities make healthier people. Find out more about other vital engineering research happening in this area.